Research
Our group’s interdisciplinary work spans biotechnology, control theory, robotics, mathematics, and machine learning. We tackle problems via experimentation, theoretical analyses, and application-focused engineering – often combining all three to link engineered biology with robotics and computational algorithms (right).
Many research projects are pursued collaboratively with academic groups at the University of Oxford and beyond, as well as with industry – particularly start-ups. The selections below provide high-level overview of ongoing research, loosely categorised under headings of Fundamentals, Robotic Platforms, and Application Areas.
Fundamentals
Fields of Science and Engineering research that underpin our work.
Synthetic Biology and Engineering Biology
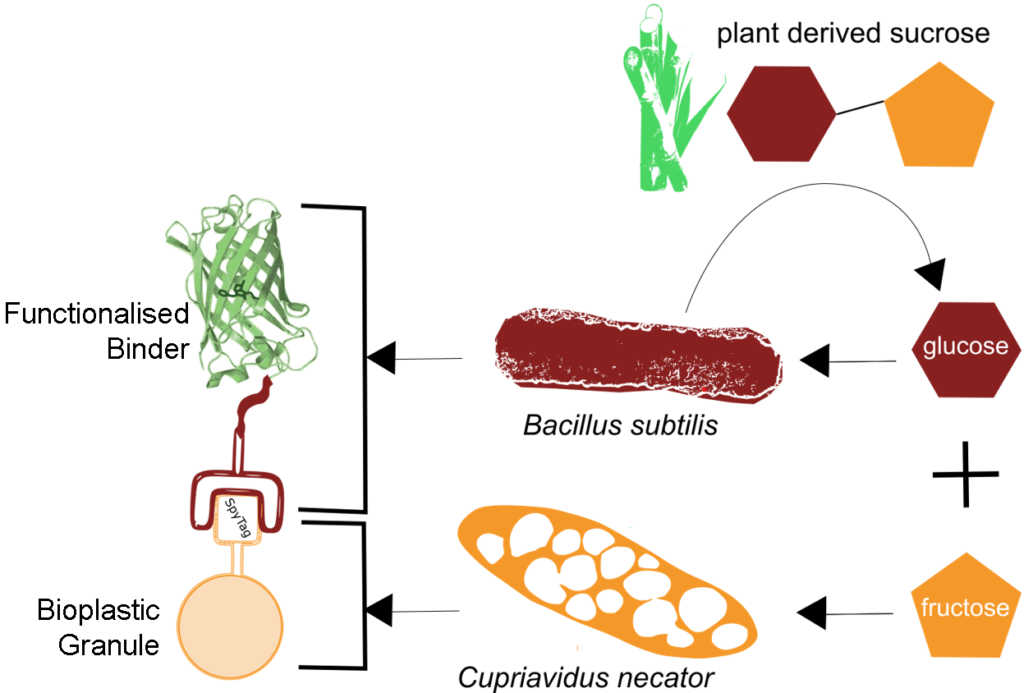
Our team’s work spans the theory, fundamentals, and applications of Synthetic Biology (or Engineering Biology) – a nascent field which aims to engineer living systems to create technologies that address some of the 21st century’s most pressing challenges. We largely focus on applications in bacteria, where we design and build novel biological functionalities (encoded in DNA) which we then test and improve in our laboratory. The example on the left shows such an application – we engineered a process in which two bacterial species cooperate to transform low-cost plant derived sugars into biodegradable plastics that are functionalised to act as sensors or catalysts for biomanufacturing.
Control Engineering
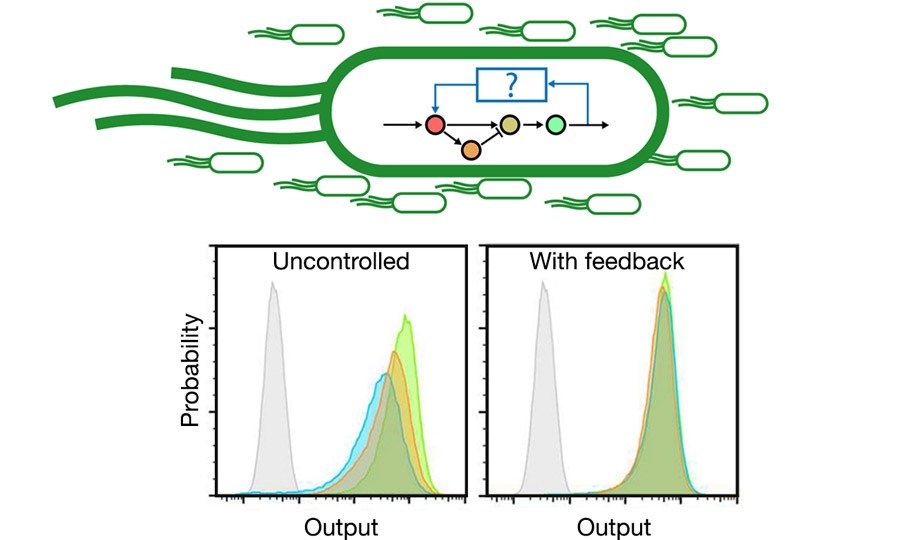
Even under controlled laboratory conditions simple biological systems can exhibit random and highly variable behaviour. This is amplified in applications beyond the laboratory, where changing environments can further impact their performance.
To address this challenge we are developing Synthetic Biological control systems that can (for example) reduce the variability of an engineered biological system’s output – helping us to create biotechnologies that are robust, predictable, and safe. For more on control and SynBio see the EEBio EPSRC Programme Grant.
AI and Machine Learning
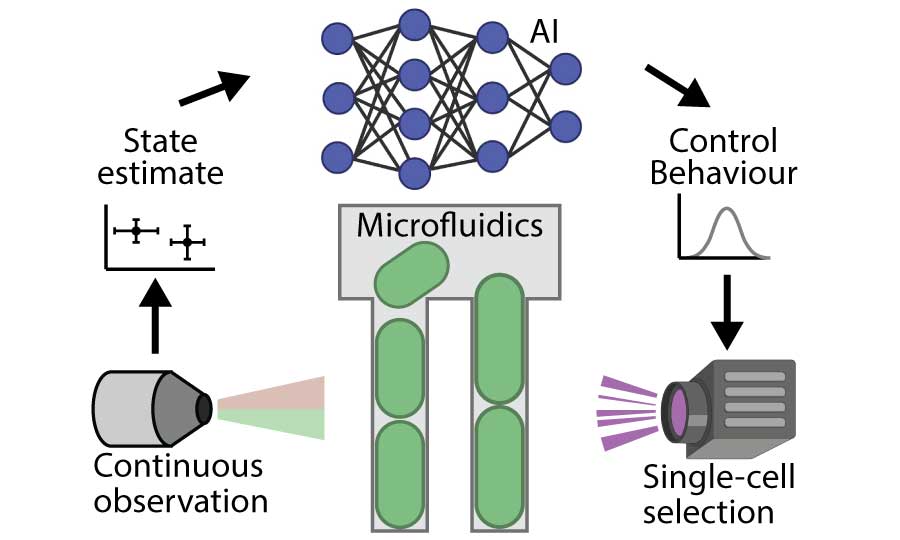
In recent years developments in Artificial Intelligence have opened the possibility of new approaches to Biotechnological research and development; new algorithms are advancing our ability to design biological systems, work with and learn from massive data-sets, and automate robotic experimental tools.
We are currently developing AI methods for a range of applications. As an example, we are building new massively-high-throughput approaches to single-cell analysis and evolution, which draw on AI tools for image processing, real-time machine learning from experimental data, and automated decision making and planning.
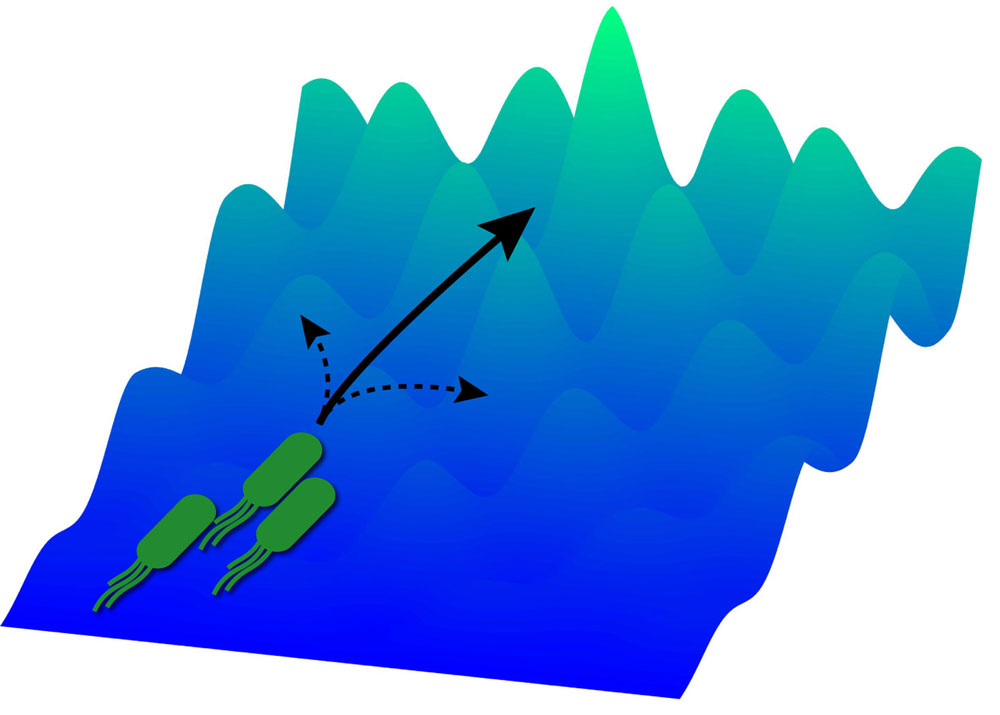
Evolutionary Methods
Evolution – nature’s own “optimisation algorithm” – is a lens through which all biology must be considered if it is to be understood. Evolutionary methods (e.g. directed evolution) are often employed to answer scientific questions, and they provide a useful alternative to the “rational design” approaches of Synthetic Biology.
We are using our unique technologies to realise new approaches to directed evolution that “close-the-loop” around experimental evolution. By dynamically (and autonomously) regulating factors such as mutagenesis and selection during an experiment we aim to accelerate and steer evolutionary trajectories.
Mathematical Biology
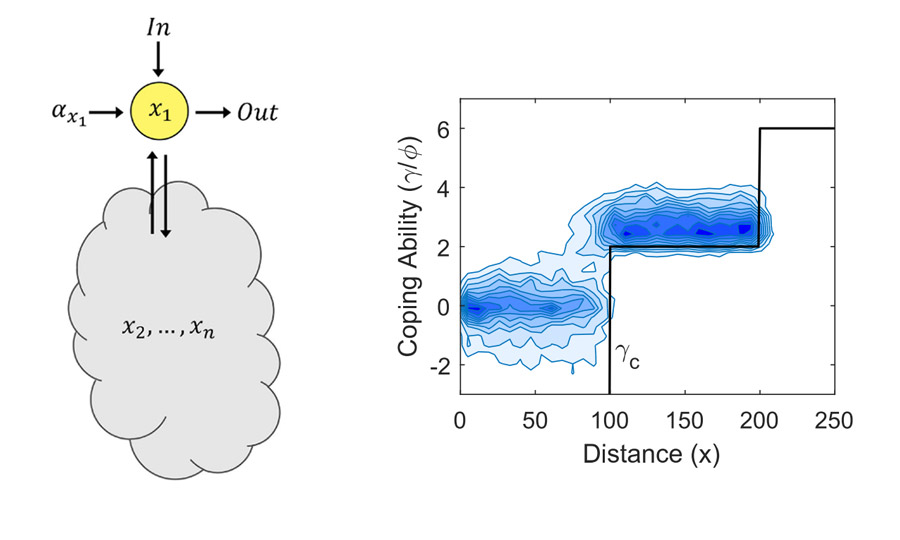
In parallel with experimental work, we frequently work on theoretical questions in biology – using mathematics and statistics to investigate or simulate systems that would be challenging to analyze in the lab.
In the past theoretical questions have spanned biological challenges including relating the structure and function of gene regulatory networks, simulating stochastic (“random”) biological phenomena such as the evolution of antibiotic resistance, and modelling how bacteria regulate their internal resource pools and growth in the presence of synthetic gene circuits.
Robotic Platforms
The frontiers of biological science and engineering are constrained by the capabilities of experimental technologies – therefore, our team creates new robotic instruments and platforms that extend our capabilities.
Robotic Bioreactors
We previously developed the Chi.Bio all-in-one bioreactor platform for automating biotech R&D. To maximise the impact of this technology, we disseminated it as an open-source not-for-profit system, allowing researchers to either build it themselves or purchase ready-made from our partner Labmaker. Due to its accessibility and unique capabilities the system has been adopted by over 50 start-up companies and a greater number of academic laboratories in the past few years. It has been applied to research challenges ranging from carbon capture to cellular agriculture to chemical biomanufacturing, and was even exhibited at the Smithsonian Museum following its use by the Open Insulin project. Learn more or purchase Chi.Bio.

Long Term Evolution Machines
Experiments that evolve microbes over many generations have contributed great advances in scientific understanding (exemplified by the famous E. coli Long Term Evolution Experiment), and also offer an engineering approach for improving and characterising new (synthetic) biotechnologies. However, traditional techniques are highly labour intensive, and limited in their ability to regulate the selective pressures (both desirable and undesirable) experienced by microbial populations. Addressing this challenge, we have created (and are continuing to develop) robotic platforms that can autonomously evolve microbes for tens of thousands of generations, with unprecedented control of selective pressures and environmental conditions.
Microscopy and Microfluidics
Many processes in bacteria can only be understood by interrogating behaviours that arise at a single-cell level. With appropriate technology it is possible to measure and manipulate many such cells in a single experiment, massively expanding the throughput of biological data collection. To this end we have developed a suite of novel microscopy hardware and software, as well as microfluidic chips to confine bacteria as they grow, to unlock massively high-throughput measurement and control of individual bacteria. This is now being applied in the lab to realise high-throughput characterisation and screening approaches, including directed evolution methods that operate at the single-cell level.

Application Areas
By combining the fundamentals and technologies developed in our team we are tackling biotechnology applications, which often includes collaboration with industry leaders, particularly start-ups.
Antimicrobial Resistance
Antimicrobial Resistance (or AMR), often referred to as the “silent pandemic”, describes the emergence and rapid proliferation of microbes that are able to resist treatment with antibiotics. Mitigating the impact of AMR will require technology development across many fields, ranging from diagnostic tools to novel drugs to better understanding of how microbes evolve. Our team is working on new technologies to identify and characterise drug resistant bacteria as part of the DARTS (Defeating Antibiotic Resistance through Transformative Solutions) project, led by Harvard Medical School and funded by the Advanced Research Projects Agency for Health (ARPA-H).
High Throughput Biodesign and Screening
Over the past five years techniques from Artificial Intelligence have unlocked a suite of new capabilities in biotechnology, enabled by the availability of large quantities of high-quality data for some biological questions. However, in areas with limited data availability, such methods have not yet found similar success. Our team is working to develop massively high-throughput characterisation and screening methods to fill this gap. This requires us to develop full-stack technologies including algorithms to optimise and automate experimental processes, biological methods for creating targeted mutant libraries, and robotic methods for characterising large libraries (i.e. millions) of variants of synthetic biological designs at single-cell level.
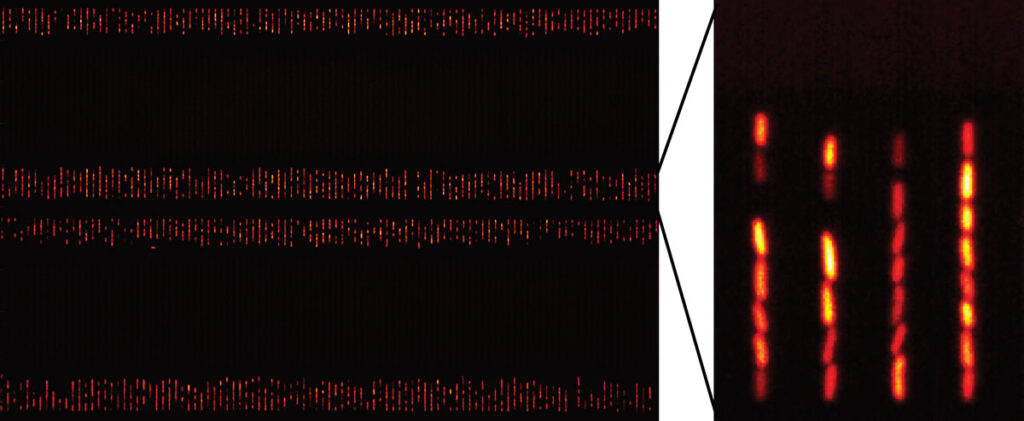
Bacteria confined in vertical channels of a “Mother Machine” microfluidic chip. On the left, one part of a single microscope field-of-view that can observe hundreds of variants. On the right, close-up of individual bacterial lineages expressing variable amounts of a fluorescent protein.
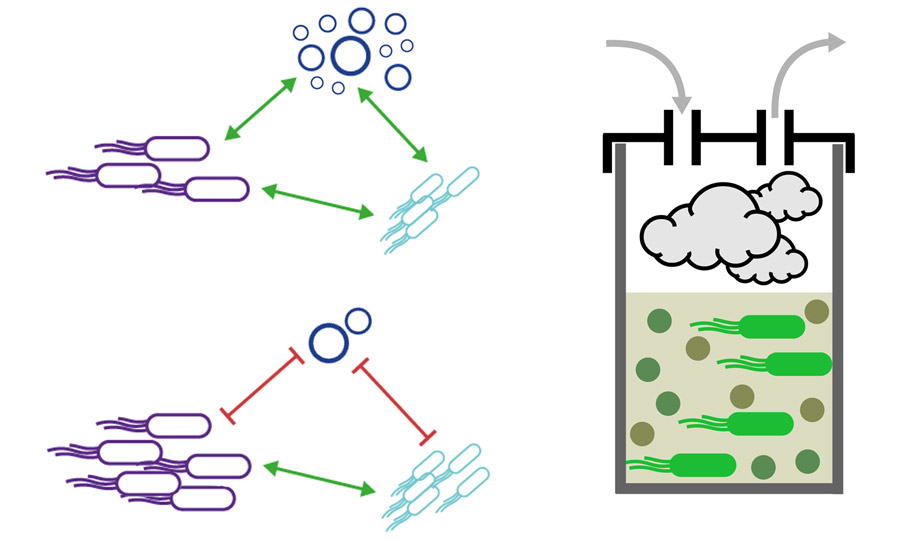
Cellular Communities
Cellular communities are complex ecosystems consisting of multiple microbial species which live in close proximity, interacting both chemically and physically. Such communities are found widely in nature and play important roles in our health (e.g. the human microbiome is a cellular community) and many biotechnologies.
We are developing and applying novel methods (both biology- and hardware-based) for real-time measurement, control, and manipulation of cellular communities for applications in biomanufacturing and bioprocess engineering.
Novel Biosensors, Reporters, and Actuators
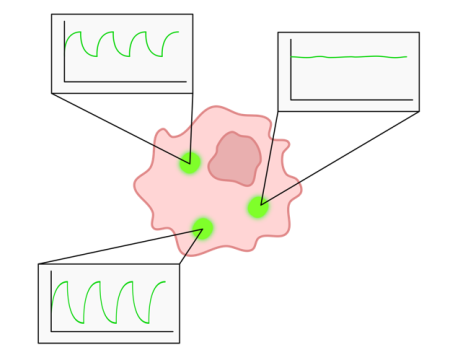
Using our techniques for high-throughput biodesign and screening we are creating new biosensor and reporter technologies, with applications in medicine, environmental sensing, and as general enablers for biotech research. For example, we use light-responsive biosystems (e.g. Optogenetics) to allow interfacing of microbial populations or single-cells with external computational control. As another example, we are developing reporters in which quantum mechanical effects lead to fluctuations in fluorescence in the presence of magnetic fields (image on right); these can be used to realise new multiplexed imaging modalities and improve measurement fidelity in noisy environments.

Biotechnology and Biomedicine
Beyond the above, we work with collaborators on interdisciplinary projects involving biotechnology, biotechnology, and mathematics. This includes advising start-up companies in industries including Cellular Agriculture, AMR, and Carbon Capture, as well as not-for-profit innovation with past projects including the OxVent Covid-19 Ventilator.

